- The Language of Nerves — Understanding the Different Types of Nerve Fibers
- When Pain Persists — The Science of Central Sensitization
- The Brain’s Superpower — How Neuroplasticity Shapes Pain (and Healing)
- Scrambler Therapy — Rewriting the Pain Story Through Neuroplasticity
- Who’s a Good Candidate for Scrambler Therapy ?
- Medications That May Interfere With Scrambler Therapy
The Language of Nerves — Understanding the Different Types of Nerve Fibers
When we experience a stubbed toe, a warm blanket, or the sting of a paper cut, it’s all thanks to our peripheral nervous system — the vast network of nerves that carries signals between the body and the brain. But not all nerves are the same. Some are fast, some slow. Some tell us something is pleasant. Others scream in pain.
To understand chronic pain—and how therapies like Scrambler Therapy work—we must first understand the different types of nerve fibers and what roles they play.
The Basics: How Nerves Transmit Signals
Nerve fibers transmit electrical impulses, called action potentials, from sensory receptors in the skin and organs to the spinal cord and brain. These fibers are categorized by:
- Diameter(larger = faster)
- Myelination (a fatty sheath that speeds transmission)
- Conduction velocity
- Function
Let’s meet the key players:
A-Beta Fibers: The Gentle Giants
- Large diameter
- Heavily myelinated
- Very fast(35–90 m/s)
- Function: Light touch, vibration, proprioception
These fibers are your “good news” nerves. They tell your brain things like “your hand is on something soft” or “you’re standing upright.” Under normal conditions, they don’t carry pain signals
A-Delta Fibers: The First Alarm Responders
- Small diameter
- Lightly myelinated
- Moderate speed (5–35 m/s)
- Function: Sharp, acute pain and temperature (cold)
These fibers act quickly to warn you of immediate, localized threats —like a pinprick or touching something sharp.
C Fibers: The Smoldering Embers
- Very small diameter
- Unmyelinated
- Slow (0.5–2 m/s);
- Function: Dull, aching, burning pain; warmth;
These fibers carry chronic or slow-onset pain. They’re responsible for the deep, throbbing ache after an injury—or the ongoing pain of neuropathy. Because they’re unmyelinated and slow, their signals arrive late and linger.
Why Does This Matter?
Chronic pain often involves overactivation or dysfunction of C and A-delta fibers. Understanding their behavior helps us identify how pain becomes chronic—and how we might reverse it.
Key clinical takeaways:
- Neuropathic pain often involves sensitized or damaged C-fibers.;
- Referred pain can involve abnormal cross-talk between different types of fibers.;
- Touch and vibration therapies often target A-beta fibers to modulate pain.;
Beyond Sensation: Nerve Fiber Plasticity
These fibers don’t just transmit signals—they change over time. With chronic pain:
- C-fibers may become hyperresponsive.;
- A-beta fibers (normally non-painful) may start carrying pain signals through “crossed wiring” at the spinal cord level.;
When Pain Persists — The Science of Central Sensitization
Imagine burning your hand on the stove. The pain is immediate, sharp, and appropriate. But what if, long after the burn has healed, simply touching your skin still caused pain—or worse, the pain spread beyond the original site and intensified over time?
This is the hallmark of a process called central sensitization —a rewiring of the nervous system that amplifies pain signals. It’s a key player in many chronic pain conditions, and understanding how it works helps us target it with treatments like Scrambler Therapy.
What Is Central Sensitization?
Central sensitization is a state in which the central nervous system (CNS)—particularly the spinal cord and brain—becomes hypersensitive to sensory input.
It occurs when:
- Normal sensory input is perceived as painful (allodynia);
- Painful input is perceived as more intense than it should be (hyperalgesia);
In essence, the volume dial on pain is turned up—even in the absence of ongoing injury.
The Biology Behind It
Let’s explore how this amplification occurs:
1. Wind-Up in the Spinal Cord
Repeated stimulation of C-fibers leads to persistent activation of dorsal horn neurons in the spinal cord. Over time, this causes:
- Increased release of neurotransmitters like glutamate and substance P
- Increased excitability of spinal neurons;
- Lower thresholds for pain signal transmission;
This is known as “wind-up”—a progressive, activity-dependent increase in pain sensitivity.
2. NMDA Receptor Activation
NMDA (N-methyl-D-aspartate) receptors in the spinal cord are critical for learning and memory—but in chronic pain, they become hijacked.
- Prolonged activation of NMDA receptors makes spinal neurons more responsive to input.;
- Even gentle stimuli (like touch) can trigger strong pain responses.;
This contributes to neuronal plasticity that favors pain persistence.
3. Loss of Inhibition
Normally, inhibitory neurons and neurotransmitters like GABA and glycine help “gate” pain signals at the spinal level. But in central sensitization:
- These inhibitory pathways are diminished or dysfunctional
- The CNS loses its natural “brakes,” allowing pain to run unchecked;
4. Glial Cell Activation and Neuroinflammation
Support cells in the CNS called glia (microglia and astrocytes) respond to injury and inflammation by releasing:
- Cytokines (pro-inflammatory)
- Prostanoids
- Chemokines
This creates a feed-forward loop of inflammation that sustains and amplifies pain signals over time.
Clinical Manifestations of Central Sensitization
Patients with central sensitization often describe:
- Widespread pain
- Fatigue and poor sleep
- Sensitivity to touch, light, sound, or temperature
- Brain fog or cognitive issues
This is a feature of many chronic pain syndromes, including:
- Fibromyalgia
- Complex regional pain syndrome (CRPS)
- Chronic low back pain
- Post-surgical pain syndromes
- Migraine
- Irritable bowel syndrome
The Brain’s Role
It’s not just the spinal cord—the brain also becomes involved:
- Functional MRI studies show increased activity in pain-processing areas (insula, anterior cingulate cortex, prefrontal cortex).
- The brain starts to anticipate pain, reinforcing its presence through attention, emotion, and memory loops.
Over time, the entire pain matrix of the brain becomes hyper-reactive—a concept known as central amplification.
Why Is This So Important?
Because if pain is being driven by central changes, then:
- Treating only the peripheral cause (like a bulging disc or nerve injury) may not relieve the pain.
- Medications alone (especially opioids) often don’t help and may even worsen the sensitivity.
- We need approaches that target the nervous system itself—and that’s where neuromodulation comes in.
The Brain’s Superpower — How Neuroplasticity Shapes Pain (and Healing)
If you’ve ever learned to ride a bike, speak a new language, or recover after a stroke, you’ve experienced neuroplasticity—the brain and nervous system’s ability to change and adapt over time.
Neuroplasticity is the cornerstone of healing—but it can also be a double-edged sword. In chronic pain, this power can reinforce suffering. But the same mechanisms that entrench pain can also unwind it, if we guide them properly.
In this post, we explore what neuroplasticity is, how it contributes to both chronic pain and recovery, and why it’s a central pillar in treatments like Scrambler Therapy.
What Is Neuroplasticity?
Neuroplasticity refers to the ability of the nervous system—brain, spinal cord, and peripheral nerves—to:
- Form new connections;
- Strengthen or weaken existing pathways;
- Reassign functions to different regions;
- Recover after injury or trauma;
It’s how we learn. How we form habits. How we adapt to new environments. And, importantly, it’s how the nervous system remembers pain.
How Pain Rewires the Nervous System
When pain becomes chronic, it’s no longer just a signal—it becomes a pattern. A loop. A reflex that gets reinforced over time. Here’s how neuroplasticity plays into it:
1. Synaptic Plasticity
Each time pain signals are sent through a neural circuit, the connections get stronger. This process, called long-term potentiation (LTP), is like practicing a piano scale—the more you repeat it, the more ingrained it becomes.
In chronic pain:
- Pain pathways are strengthened.
- “Non-pain” signals can start to trigger pain (sensory remapping).
- The brain becomes more efficient at producing the experience of pain.
2. Maladaptive Neuroplasticity
Neuroplasticity is neutral—it simply adapts to repeated input. But that adaptation can be helpful or harmful.
- In phantom limb pain, the brain remaps the missing limb’s area to nearby body parts, creating “ghost pain.”
- In fibromyalgia, regions of the brain associated with pain processing show increased activity and connectivity, even without injury.
The brain literally learns pain.
3. Central Sensitization Is Neuroplasticity Gone Wrong
As discussed in the last post, central sensitization is a plastic change where the CNS becomes hyper-responsive.
This involves:
- Upregulation of excitatory neurotransmitters
- Downregulation of inhibition
- Structural changes in the spinal cord and brain
The longer pain persists, the deeper these changes go. That’s why early intervention—and reversal—is key.
The Good News: Neuroplasticity Can Work In Your Favor
Just as the brain can learn pain, it can also unlearn it.
This involves:
- Strengthening non-pain pathways
- Reestablishing normal sensory input
- Dampening overactive pain circuits
- Building new sensory associations
Techniques that leverage positive neuroplasticity include:
- Graded motor imagery
- Mirror therapy
- Mindfulness and CBT
- Neuromodulation techniques like Scrambler Therapy
Brain Areas Involved in Pain Plasticity
Pain is not processed in one spot—it’s distributed across the pain matrix, including:
- Somatosensory cortex (location and quality)
- Anterior cingulate cortex (emotional response)
- Insula (internal body awareness)
- Prefrontal cortex (attention, meaning)
- Amygdala and hippocampus (memory and emotion)
These regions change structurally and functionally in people with chronic pain.
But they can also normalize when pain is successfully treated. This is the goal of any therapy that targets the neural roots of pain.
Connecting the Dots
We now understand:
- Nerve fibers transmit pain based on their size and function (Post 1).
- Central sensitization amplifies that pain via plastic changes in the spinal cord and brain (Post 2).
- Neuroplasticity underlies both the worsening—and potential reversal—of pain
Scrambler Therapy — Rewriting the Pain Story Through Neuroplasticity
Over the past three posts, we’ve explored how the nervous system communicates pain (Post 1), how chronic pain can hijack the system (Post 2), and how neuroplasticity drives both suffering and recovery (Post 3).
Now, we turn to a powerful and innovative treatment: Scrambler Therapy—a non-invasive neuromodulation technique that leverages everything we’ve discussed to reset the nervous system’s perception of pain.
Pain Is a Language—Scrambler Therapy Teaches a New One
The central idea of Scrambler Therapy is this: if chronic pain is the result of faulty information sent by the nervous system, we can overwrite it with healthy, non-pain information.
Rather than blocking pain signals like medications or numbing nerves like local anesthetics, Scrambler Therapy replaces pain signals with synthetic “non-pain” messages that the brain learns to accept as normal.
Think of it as teaching the brain a new sensory language—one that says, “You’re okay now.”
How Scrambler Therapy Works (Mechanistically)
1. Targeting C-Fibers (the Slow Pain Pathways)
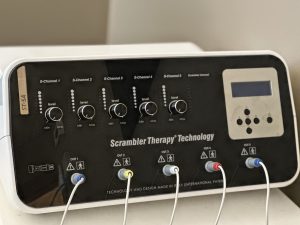
Scrambler Therapy uses surface electrodes placed near—but not on—the painful area to stimulate the skin and underlying nerves.
The signals are:
- Low-frequency
- Variable (non-repetitive and non-linear)
- Specifically tuned to mimic healthy nerve signaling
These signals travel through the same C-fibers and A-delta fibers that normally carry chronic pain—but instead of transmitting pain, they transmit information that the brain interprets as non-threatening.
2. Deactivating Central Sensitization
With repeated treatments, Scrambler Therapy helps:
- Interrupt the loop of central sensitization in the spinal cord
- Reduce activation of NMDA receptors
- Re-establish inhibitory control (via GABAergic and glycinergic neurons)
This “turns down the volume” of pain at its central source.
3. Rewiring the Brain Through Neuroplasticity
As the therapy is repeated over 10–12 sessions, it leverages positive neuroplasticity:
- Strengthens non-pain sensory pathways
- Weakens chronic pain loops
- Teaches the brain that previously painful stimuli are now safe
Many patients report long-term pain relief, even after sessions are complete—evidence that the brain has been retrained.
Clinical Support and Results
Numerous studies and clinical trials support Scrambler Therapy’s efficacy:
- Cancer-related neuropathy (CIPN): Significant pain reduction in patients with chemotherapy-induced nerve damage.
- Failed back surgery syndrome, postherpetic neuralgia, diabetic neuropathy: Reported pain relief often exceeds what is achieved with medications.
- RCTs show durable results, with many patients reporting relief lasting weeks to months after treatment completion.
Importantly:
- No drugs
- No sedation
- No known serious side effects
Why Scrambler Therapy Is Different
| Feature | Scrambler Therapy | Traditional Pain Treatments |
| Mechanism | Information replacement | Numbing, blocking, or dulling pain |
| Target | C-fibers, spinal cord, brain | Peripheral nerves, inflammatory mediators |
| Goal | Neuroplastic reset | Temporary symptom control |
| Duration of Relief | Often long-lasting (weeks to months) | Short-term (hours to days) |
| Side Effects | Minimal to none | Often significant |
Ideal Candidates
Scrambler Therapy is especially useful for:
- Neuropathic pain (e.g. diabetic, post-surgical, postherpetic)
- CRPS
- CIPN
- Phantom limb pain
- Failed back surgery syndrome
- Pain after shingles or nerve injury
It’s best for patients who:
- Have not responded to conventional medications
- Prefer non-invasive, drug-free options
- Are medically complex or opioid-intolerant
Putting It All Together
Let’s recap how Scrambler Therapy aligns with the biology we’ve covered:
| Concept | Problem | Scrambler Therapy’s Solution |
| Nerve Fiber Dysfunction | C-fibers overfiring | Sends healthy signals via same pathways |
| Central Sensitization | Amplified spinal cord response | Disrupts pain wind-up and restores balance |
| Neuroplasticity | Maladaptive pain learning | Retrains brain and spinal cord to forget pain |
The Future of Pain Management?
Scrambler Therapy is part of a broader movement in medicine—targeting the nervous system as a dynamic, trainable network, rather than a static, damaged machine.
It’s a hopeful therapy, not because it masks pain, but because it respects the nervous system’s ability to heal—when given the right input.
Final Thoughts
Pain that no longer serves a purpose doesn’t have to become your identity. By understanding how pain works at the level of nerve fibers, the spinal cord, and the brain, we open the door to smarter, gentler, and more effective treatments.
Scrambler Therapy isn’t magic—it’s science. And it just might help your nervous system remember what comfort feels like.
Who’s a Good Candidate for Scrambler Therapy — And Which Medications Might Interfere?
Scrambler Therapy is a promising, non-invasive treatment for chronic neuropathic pain. But like any therapy, it works best under certain conditions—and for certain patients.
In this post, we’ll explore:
- Who makes an ideal candidate for Scrambler Therapy;
- Which medications may interfere with its success;
- Why these medications may need to be adjusted or tapered during treatment;
Ideal Candidates for Scrambler Therapy
Scrambler Therapy can benefit a wide range of patients—but its best results are seen in patients with chronic neuropathic pain that is:
- Localized or regional (not widespread fibromyalgia-type pain);
- Persistent despite medication;
- Present for at least 3 months;
- Not caused by ongoing active tissue damage;
Here’s who tends to benefit the most:
Conditions That Respond Well
- Chemotherapy-induced peripheral neuropathy (CIPN);
- Post-surgical neuropathic pain (e.g., mastectomy, hernia repair);
- Postherpetic neuralgia;
- Diabetic peripheral neuropathy<;
- Failed back surgery syndrome;
- CRPS (Complex Regional Pain Syndrome);
- Phantom limb pain;
- Radiculopathy (especially after the acute phase);
Patient Characteristics That Predict Success
- Pain that’s neuropathic in nature (burning, tingling, shooting);
- Pain that’s static or improving, not worsening from a progressive condition;
- Skin intact over the treatment area (no open wounds or ulcers);
- Able to provide feedback during treatment sessions (Scrambler is interactive);
- Willing and able to attend daily sessions for 10–12 days;
Who May Not Be a Good Candidate?
Scrambler Therapy is not recommended for:
- Patients with mechanical or inflammatory pain only (e.g., arthritis without neuropathy);
- People with pacemakers or implanted defibrillators;
- Patients with significant cognitive impairment who can’t give reliable feedback;
- Areas with no sensation (Scrambler relies on intact sensory pathways);
- Active, worsening disease processes like spreading cancer unless neuropathic pain is the main symptom
Medications That May Interfere With Scrambler Therapy
While many medications can be continued during treatment, some drugs may reduce the effectiveness of Scrambler Therapy by blunting the nervous system’s ability to respond and rewire.
These medications include:
1. Long-Acting Opioids (e.g., OxyContin, MS Contin, fentanyl patches)
- Why they interfere: Opioids dull the nervous system’s responsiveness and may impair the feedback loop that Scrambler Therapy relies on to reprogram nerve signals.;
- What to do: Tapering or pausing long-acting opioids is often recommended prior to or during therapy, if clinically appropriate. Short-acting opioids may be used as needed, but should be spaced several hours away from treatment sessions.
2. Ketamine and NMDA Antagonists
- Why they interfere: Scrambler Therapy engages NMDA receptor modulation to achieve central desensitization. Ketamine or other NMDA antagonists may interfere with this reprogramming process.;
- What to do: Delay Scrambler Therapy until after ketamine infusions are completed, or allow a washout period.;
3. Benzodiazepines (e.g., clonazepam, lorazepam)
- Why they interfere: These suppress CNS activity and may impair neuroplasticity, making it harder for the therapy to take hold.;
- What to do: If used regularly, consider tapering under supervision. Occasional use may be acceptable, but should be spaced several hours from treatment.;
4. High-Dose Gabapentinoids (gabapentin, pregabalin)
- Why they interfere: While these drugs treat neuropathic pain, high doses may dull sensory input and hinder feedback during therapy sessions.;
- What to do: If the patient is on very high doses, a dose reduction may be considered. However, many patients still benefit from Scrambler Therapy while on gabapentinoids, especially at moderate doses.;
Medications That Are Typically Compatible
- Antidepressants (e.g., duloxetine, amitriptyline)p;
- NSAIDs
- Muscle relaxants (e.g., tizanidine, cyclobenzaprine);
- Acetaminophen;
- Topical agents (lidocaine patches, capsaicin);
- Low-dose opioids timed well outside of treatment sessions;